Many drugs are sensitive to light, and fiber optics can rapidly reduce their effectiveness. Therefore, not only during storage, but also during infusion, infusion catheters made of light-proof materials must be used. To this end, Qingdao Primetech Plastics specially customized a light-shielding functional masterbatch for a high-end medical device manufacturer.
Case name (Example)
●Product Application (Application): Light-proof infusion tube (implanted catheter extension tube)
●Product name (Product): Medical functional masterbatch - light-proof masterbatch
●Product model (Size): K70062TU-M
●Manufacturer (OEM): A high-end medical device manufacturer in Zhengzhou
●End user:
●First order: 100kg
●R&D service team: engineer Kong (sales manager), engineer Li (R&D),engineer Wang (color)
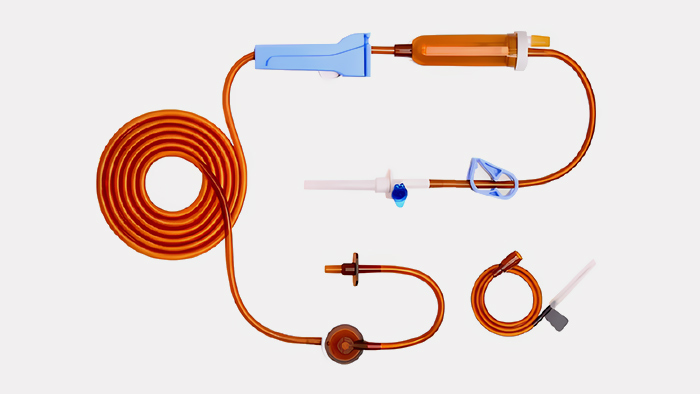
Application Requirements:
1. Conform to the national standard of medical products
2. The addition ratio is controlled within 10%
3. The product thickness is 0.2-0.3mm within the wavelength range of 290-450mm, and the light transmittance is ≤15%
4. Color brown
5. Uniform particle size requires about φ3*3mm
Reasons for success:
1. Accurate understanding of customer requirements;
2. The company has always had experience in making medical masterbatch, and its engineers have researched functional masterbatch;
3. The product can meet all customer requirements.

Qingdao primetech Plastic has 16 years of modified production experience, focusing on one-to-one customized development (products include flame-retardant modified materials, PC/ABS alloys, medical grade masterbatch, etc.) and mature formulas to ensure that products can accurately match customer requirements . It solves the cost waste caused by performance overflow and provides you with more suitable modified materials. If you also have needs for flame-retardant HIPS, please call us for consultation. You can also consult/leave messages online to communicate directly with customer service.

 English
English














